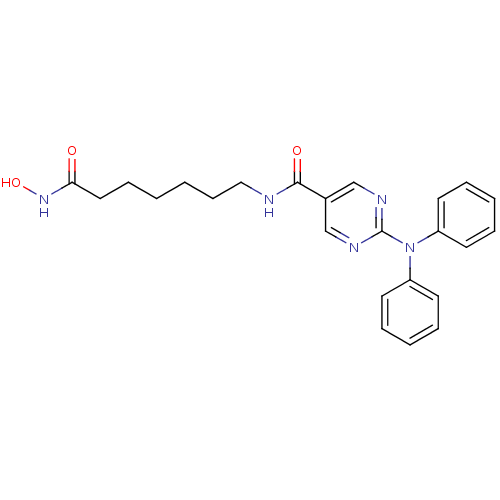
BDBM50439674 RICOLINOSTAT::US10858323, Compound 2::US11207431, Example E::US20230414581, Compound 43::US8609678, 2-(diphenylamino)-N-(7-(hydroxyamino)-7-oxoheptyl)pyrimidine-5-carboxamide [26]
SMILES ONC(=O)CCCCCCNC(=O)c1cnc(nc1)N(c1ccccc1)c1ccccc1
InChI Key InChIKey=QGZYDVAGYRLSKP-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50439674
Found 9 hits for monomerid = 50439674
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of recombinant HDAC9 (unknown origin) using AMC labeled AC-peptide as substrate incubated for 1 hr by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of HDAC9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMAssay Description:Inhibition of HDAC9 (unknown origin) using MAZ-1675 as substrate preincubated for 10 mins followed by substrate addition and shaken for 60 secs and m...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMAssay Description:Inhibition of HDAC9 (unknown origin) using MAZ-1675 as substrate preincubated for 10 mins followed by substrate addition and shaken for 60 secsMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:In vitro antagonistic activity against kinin-induced rabbit jugular vein contraction.More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMAssay Description:IC50 measurements were conducted by BPS Biosciences (Table 1) or by Nanosyn (Table 1A) with an established fluorescence assay. More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of HDAC9 (unknown origin) using fluorescent peptide substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+3nMAssay Description:Inhibition of recombinant HDAC9 (unknown origin) using Boc-Lys (trifluoroacetyl) AMC as substrate preincubated for 1 hr followed by substrate additio...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of HDAC9 (unknown origin) using MAZ-1675 as substrate preincubated for 10 mins followed by substrate addition by microtiter plate reader a...More data for this Ligand-Target Pair